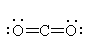 the central carbon atom giving it a linear electron geometry. With no lone pairs, it also has a linear molecular geometry. |
|
|
|
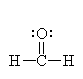
It has three groups of electrons around
|
|
|
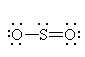
It has three groups of electrons around the central sulfur atom giving it a trigonal planar electron geometry. With one lone pair, it has a bent molecular geometry. Because it only has one lone pair, it slightly different than the bent shape of water. |
|
|
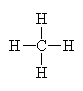
It has four groups of electrons around the central carbon atom giving it a tetrahedral electron geometry. With no lone pairs, it also has a tetrahedral molecular geometry. |
This is ammonia (NH3)
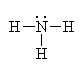
It has four groups of electrons around the central nitrogen atom giving it a tetrahedral electron geometry. With one lone pair, it has a trigonal pyramidal molecular geometry. |
|
| This is water (H2O)
It has four groups of electrons around the central oxygen atom giving it a tetrahedral electron geometry. With two lone pairs, it has a bent molecular geometry. Because it has two lone pairs, it slightly different than the bent shape of SO2. |
|
|
|
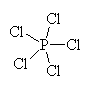
It has five groups of electrons around the central phosphorous atom giving it a trigonal bipyramidal electron geometry. With no lone pairs, it also has a trigonal bipyramidal molecular geometry. |
|
|
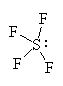
It has five groups of electrons around the central sulfur atom giving it a trigonal bipyramidal electron geometry. With one lone pair, it has a see saw molecular geometry. |
|
|
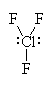
It has five groups of electrons around the central sulfur atom giving it a trigonal bipyramidal electron geometry. With two lone pairs, it has a tee shaped molecular geometry. |
|
|
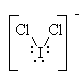
It has five groups of electrons around the central iodine atom giving it a trigonal bipyramidal electron geometry. With three lone pairs, it has a linear molecular geometry. |
|
|
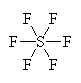
It has six groups of electrons around the central sulfur atom giving it an octahedral electron geometry. With no lone pairs, it also has a octahedral molecular geometry. |
|
|
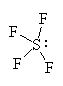
It has six groups of electrons around the central bromine atom giving it an octahedral electron geometry. With one lone pair, it has a square pyramidal molecular geometry. |
|
|
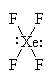
It has six groups of electrons around the central xenon atom giving it an octahedral electron geometry. With two lone pairs, it has a square planar molecular geometry. |