| Quantum Mechanics in Three dimension (Part II - Atomic Structure) |
In Part I we studied the Hydrogen atom, learned about the different quantum numbers. In Part II we will study the effect of magnetic field on the energy levels (as an example say of the hydrogen atom). We will learn about a new quantum number - the 'spin' quantum number. Finally we will study the rules governing the electronic configurations of the elements in the periodic table.
In Part I we found that the acceptable wavefunctions of the hydrogen atom can be found only if the energy E is restricted to be one of the following special values:
En = -(me4/2hbar2)(Z2/n2), n=1,2,3,....
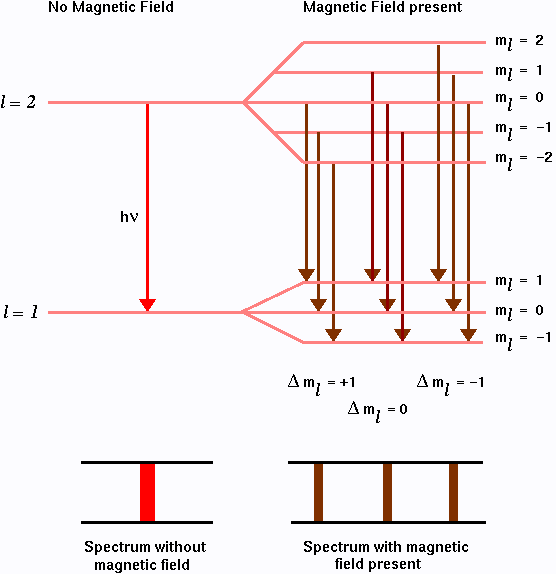
Note that the allowed energies depend only on the principal quantum number n. Keeping n fixed but allowing l [l can vary from 0 to (n-1)] and ml [ml varies from -l,-(l-1),...,0,...(l-1),l] to vary the energy doesnot change. This is another example of degeneracy. States with fixed n have same energies for different l and ml. But this degeneracy can be partially removed by applying an external magnetic field. This is known as the Zeeman effect.
Orbital Magnetism And The Zeeman Effect :
It is known from classical electromagnetic theory (Maxwell's equations) that a rotating charged body gives rise to a magnetic field. So an electron orbiting the nucleus of an atom generates a magnetic field. The atom thus behaves like a tiny magnet with magnetic moment m. It can be easily shown that for a circulating charge (of charge q and mass m) the magnetic moment m is proportional to the orbital angular momentum L. Moreover, m and L are in the same direction. Mathematically,
m = (q/2m) L.
The constant (q/2m) is called the gyromagnetic ratio. For the case of an electron rotating around the nuclues the magnetic moment is in opposite direction to the angular momentum as the charge of the electron is negative. So in case of electrons we have,
m = (-e/2me) L.
Considering only the z-component we get,
mz = (-e/2me) Lz = -(e hbar/2me) ml = -mB ml, (As Lz = ml hbar)
where, mB = e hbar/2me = 9.274 x 10-24 J/T is called the Bohr magneton. It is a natural unit for the atomic moment. Thus we can see that mz can take only special values.
The interaction of an atom with an applied magnetic field depends ont he size and orientation of the atom's magnetic moment. With the application of a magnetic field B, the atom experiences a torque t,
t = m x B.
If the applied magnetic field is in the z-direction the atom precesses around the field direction with a frequency wL known as the Larmor frequency,
wL = (e/2me) B.
It can be shown from electromagnetic theory that the energy of a magnetic dipole in an external magnetic field B is,
U = -m.B = (e/2me) L.B = (eB/2me)Lz = hbar wL ml.
Equation above shows that the magnetic energy of an atomic electron depends on the magnetic quantum number ml and hence, is quantized. The total energy of this electron is the magnetic energy U plus the energy it had in the absence of an applied field (E0). So we have,
E = E0 + hbar wL ml.
For atomic hydrogen, E0 depends only on the principal quantum number n. We can see that in presence of the magnetic field the energy levels are no longer degenerate. For example, for the first excited state n=2, l=1, ml can take three values -1, 0, and 1. Thus the energies are
E1 = E0 - hbar wL, E2 = E0, and E3 = E0 + hbar wL.
So the single line will split in to three equally spaced lines (thus partially lifting the degeneracy) as shown in the figure below:

The splitting of energy levels in presence of magnetic field is known as the Zeeman effect. Zeeman spectral lines and the underlying atomic transition that give rise to them for an electron follows from the selection rules discussed before (Part I). The allowed atomic transitions from l=2 to l=1 level are shown in the figure above.
Finally, even the splitting of an emission line into a triplet of equally spaced lines as predicted, which is called the normal Zeeman effect is not observed experimentally. In the experiment one observes for than three spectral lines (four, six or even more). This is the anomalous Zeeman effect which is due to the existence of the electron spin (to be discussed below).
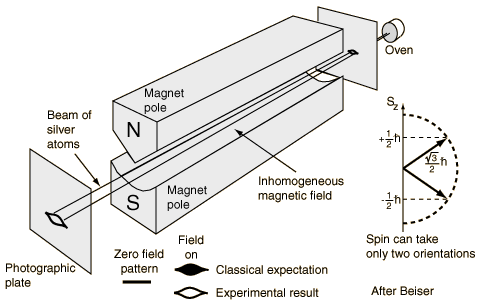
The magnetic moment seen in the Stern-Gerlach experiment is attributed to the spin of the outermost electron in silver. The result of the Stern-Gerlach experiment is:
Sz = ms hbar. [ms = 1/2 or -1/2 for electrons].
ms = +1/2 refers to the up spin case and ms = -1/2 refers to the spin down case. Stern-Gerlach experiment for l=1 electrons are shown below -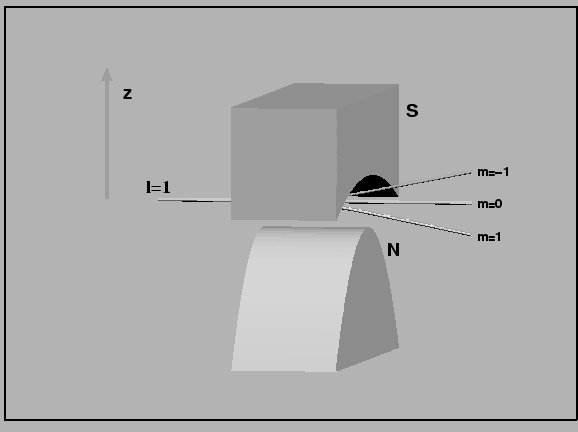
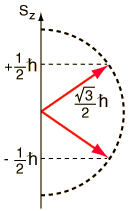
The spin angular momentum also exhibits space quantization. The figure below shows the two allowed orientations of the spin vector S for a spin 1/2 particle (e.g. electron):
Total Angular Momentum:
The electron thus have both orbital (L) and spin angular momentum (S). The existence of both orbital and spin moments for the electrons lead to their mutual interaction known as the spin-orbit interaction. The coupling of spin and orbital moments implies that neither L or S is conserved separetly. But the total angular momentum (J) which is just the sum of L and S i.e. J = L + S is conserved. Thus |J| and |Jz| are quantized and only takes the following values:
|J| = [j(j+1)]1/2 hbar, Jz = mj hbar with mj = j, j-1,...., -j.
Permissible values for the total angular quantum number j are
j = l+s, l+s-1, l+s-2, ....,|l-s|.
The existence of spin requires that the state of an atomic electron has to be specified with four quantum numbers l, s, ml, and ms. If the spin-orbit interaction is taken into account, ml and ms are replaced by j and mj. In either case four quantum numbers are required, one for each of the four degrees of freedom possessed by a single electron.
Exchange Symmetry and the Pauli exclusion principle:
Consider a system where two or more electrons are present. It is natural to ask "How many electrons in an atom can have the same four quantum numbers in the same state?" According to Pauli "no two electrons in an atom can have the same set of quantum numbers." This is the famous Pauli's exclusion principle. The exclusion principle follows from tha fact that the electrons are identical particles - it is not possible to distinguish between two electrons.
Exchange Symmetry
Let us consider the two-particle wavefunction Y(r1, r2) which depends on both the particles 1 (at position r1) and 2 (at position r2). |Y(r1, r2)|2 then represents the probability density for finding one electron at r1 and the other electron at r2. Now if the particles are indistinguishable a formal interchange of particles produce no observable effects. Mathematically,
|Y(r1, r2)| = |Y(r2, r1)|
Such a wavefunction then said to have a exchange symmetry. But the wavefunction itself may be either even or odd under exchange symmetry i.e. we can have two following cases:
Y(r1, r2) = + Y(r2, r1) (Bosons)
Y(r1, r2) = - Y(r2, r1) (Fermions)
The particles with even exchange symmetry are known as bosons (we will see later that they obey Bose-Einstein statistics). Photons (quanta of electromagnetic wave), phonons (quanta of lattice vibrations), pions etc. are examples of bosons. Bosons have integer spins.
The particles with odd exchange symmetry are known as fermions (they obey Fermi-Dirac statistics). Electrons, protons, neutrinos, etc are examples of fermions. Fermions have half-integer spins.
Electron Configurations & The Periodic Table:
The Relative Energies of
Atomic Orbitals
Because of the force of attraction between objects of opposite charge, the most important
factor influencing the energy of an orbital is its size and therefore the value of the
principal quantum number, n. For an atom that contains only one electron,
there is no difference between the energies of the different subshells within a shell. The
3s, 3p, and 3d orbitals, for example,
have the same energy in a hydrogen atom. The Bohr model, which specified the energies of
orbits in terms of nothing more than the distance between the electron and the nucleus,
therefore works for this atom. The hydrogen atom is unusual, however. As soon as an atom
contains more than one electron, the different subshells no longer have the same energy.
Within a given shell, the s orbitals always have the lowest energy. The
energy of the subshells gradually becomes larger as the value of the angular quantum
number becomes larger.
Relative energies: s < p < d < f
As a result, two factors control the energy of an orbital for most atoms: the size of the orbital and its shape, as shown in the figure below.
![]()
A very simple device can be constructed to estimate the relative energies of atomic orbitals. The allowed combinations of the n and l quantum numbers are organized in a table, as shown in the figure below and arrows are drawn at 45 degree angles pointing toward the bottom left corner of the table.
![]()
The order of increasing energy of the orbitals is then read off by following these arrows, starting at the top of the first line and then proceeding on to the second, third, fourth lines, and so on. This diagram predicts the following order of increasing energy for atomic orbitals.
1s < 2s < 2p < 3s < 3p <4s < 3d <4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d
< 7p < 8s ...
Electron Configurations, the
Aufbau Principle, Degenerate Orbitals, and Hund's Rule
The electron configuration of an atom describes the orbitals occupied by electrons on the atom. The basis of this prediction is a rule known as the aufbau principle, which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital. A hydrogen atom (Z = 1) has only one electron, which goes into the lowest energy orbital, the 1s orbital. This is indicated by writing a superscript "1" after the symbol for the orbital.
H (Z = 1): 1s1
The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital.
He (Z = 2): 1s2
The third electron goes into the next orbital in the energy diagram, the 2s orbital.
Li (Z = 3): 1s2 2s1
The fourth electron fills this orbital.
Be (Z = 4): 1s2 2s2
After the 1s and 2s orbitals have been filled, the next lowest energy orbitals are the three 2p orbitals. The fifth electron therefore goes into one of these orbitals.
B (Z = 5): 1s2 2s2 2p1
When the time comes to add a sixth electron, the electron configuration is obvious.
C (Z = 6): 1s2 2s2 2p2
However, there are three orbitals in the 2p subshell. Does the second electron go into the same orbital as the first, or does it go into one of the other orbitals in this subshell? To answer this, we need to understand the concept of degenerate orbitals. By definition, orbitals are degenerate when they have the same energy. The energy of an orbital depends on both its size and its shape because the electron spends more of its time further from the nucleus of the atom as the orbital becomes larger or the shape becomes more complex. In an isolated atom, however, the energy of an orbital doesn't depend on the direction in which it points in space. Orbitals that differ only in their orientation in space, such as the 2px, 2py, and 2pz orbitals, are therefore degenerate. Electrons fill degenerate orbitals according to rules first stated by Friedrich Hund. Hund's rules can be summarized as follows. One electron is added to each of the degenerate orbitals in a subshell before two electrons are added to any orbital in the subshell. Electrons are added to a subshell with the same value of the spin quantum number until each orbital in the subshell has at least one electron.When the time comes to place two electrons into the 2p subshell we put one electron into each of two of these orbitals (The choice between the 2px, 2py, and 2pz orbitals is purely arbitrary).
C (Z = 6): 1s2 2s2 2px1 2py1
The fact that both of the electrons in the 2p subshell have the same spin quantum number can be shown by representing an electron for which s = +1/2 with an arrow pointing up and an electron for which s = -1/2 with an arrow pointing down. The electrons in the 2p orbitals on carbon can therefore be represented as follows.
![]()
When we get to
N (Z = 7), we have to put one electron into each of the three degenerate 2p orbitals.N (Z = 7): 1s2 2s2 2p3 |
|
Because each orbital in this subshell now contains one electron, the next electron added to the subshell must have the opposite spin quantum number, thereby filling one of the 2p orbitals.
O (Z = 8): 1s2 2s2 2p4 |
|
The ninth electron fills a second orbital in this subshell.
F (Z = 9): 1s2 2s2 2p5 |
|
The tenth electron completes the 2p subshell.
Ne (Z = 10): 1s2 2s2 2p6 |
|
There is something unusually stable about atoms, such as He and Ne, that have electron configurationswith filled shells of orbitals. By convention, we therefore write abbreviated electron configurations in terms of the number of electrons beyond the previous element with a filled-shell electron configuration. Electron configurations of the next two elements in the periodic table, for example, could be written as follows.
Na (Z = 11): [Ne] 3s1
Mg (Z = 12): [Ne] 3s2
The Aufbau process can be used to predict the electron configuration for an element. The actual configuration used by the element has to be determined experimentally. The experimentally determined electron configurations for the elements in the first four rows of the periodic table are given in the periodic table [].
Exceptions to Predicted Electron Configurations
There are several patterns in the electron
configurations listed in the periodic table. One of the most striking is the remarkable
level of agreement between these configurations and the configurations we would predict.
There are only two exceptions among the first 40 elements: chromium and copper.
Strict adherence to the rules of the Aufbau process would predict the following electron
configurations for chromium and copper.
Predicted electron configurations: |
Cr
(Z = 24): [Ar] 4s2 3d4 |
The experimentally determined electron configurations for these elements are slightly different.
Actual electron configurations: |
Cr (Z = 24): [Ar] 4s1 3d5 Cu (Z = 29): [Ar] 4s1 3d10 |
In each case, one electron has been transferred from the 4s orbital to a 3d orbital, even though the 3d orbitals are supposed to be at a higher level than the 4s orbital. Once we get beyond atomic number 40, the difference between the energies of adjacent orbitals is small enough that it becomes much easier to transfer an electron from one orbital to another. Most of the exceptions to the electron configuration predicted from the aufbau diagram shown earlier therefore occur among elements with atomic numbers larger than 40. Although it is tempting to focus attention on the handful of elements that have electron configurations that differ from those predicted with the Aufbau diagram, the amazing thing is that this simple diagram works for so many elements.
The figure below shows the relationship between the periodic table and the orbitals being filled during the aufbau process. The two columns on the left side of the periodic table correspond to the filling of an s orbital. The next 10 columns include elements in which the five orbitals in a d subshell are filled. The six columns on the right represent the filling of the three orbitals in a p subshell. Finally, the 14 columns at the bottom of the table correspond to the filling of the seven orbitals in an f subshell.
![]()
© Kingshuk Majumdar (2000)