Physiologically Structured Models of Rotifer Population Dynamics in a Chemostat
James N. McNair
Annis Water Resources Institute
Project description
The current state of knowledge of basic population dynamics principles is unsatisfactory on empirical as well as theoretical grounds. The main problems are a weak experimental base and a body of theory dominated by simplistic models that lack explicit biological mechanisms. In my view, there is a clear need for renewed emphasis on rigorous laboratory studies of population dynamics, for the development of physiologically structured population models whose relationship to real experimental systems is direct and unambiguous, and for vigorous interplay between these empirical and theoretical approaches.
The rotifer chemostat is an excellent experimental tool for conducting detailed laboratory studies of population dynamics. A chemostat is a constant-volume, continuous-flow culture system. Its main parts consist of at least one culture vessel (also called a reactor), a mechanism for continually mixing the culture, an input port through which fresh culture medium is continually pumped into the culture, and an output port through which culture continually washes out. Though originally developed for culturing bacteria, several workers have successfully employed the chemostat with rotifers. In this case, the culture medium pumped into the culture vessel is a unicellular algal suspension. Rotifers in the culture vessel (e.g., Brachionus calyciflorus, photo above left) ingest the algae as food. If desired, algal growth and division can be essentially stopped by maintaining the culture vessel in darkness. (See Boraas et al., 1998 for further details on the rotifer chemostat.)
Previous workers who wished to model the rotifer chemostat simply employed traditional models (or minor variants) that were originally developed for the microbial chemostat. These models fail to include any form of population structure. For example, there is no distinction between eggs, juveniles, and adults. All individuals are therefore assumed to assimilate and allocate ingested food in exactly the same way. Among other things, this means that all individuals—even neonates—are allowed to reproduce at the same rate.
McNair et al. (1998) examined several lines of published experimental evidence that make it possible to evaluate the ability of traditional unstructured chemostat models to account for observed dynamics. The evidence clearly indicates that these models are inadequate. Not surprisingly, the most damning evidence comes from experiments that introduce pronounced transient dynamics in a population initially at steady state. Such experiments are well known to be particularly effective at revealing the inner workings of a population. Dynamics computed with traditional chemostat models (e.g., the Monod-Herbert model) showed gross discrepancies from observed transient dynamics, even failing to reproduce the broadest qualitative features. Another problem with traditional chemostat models is that, because they fail to include population structure, they are incapable of addressing important lines of evidence regarding the size- and stage-structure of rotifer populations, generated by Boraas and coworkers.
How are we to improve upon the traditional chemostat models? One approach is to retain most of the basic form of these unstructured models but to introduce ad hoc, non-mechanistic devices such as time-delays in order to enrich their transient dynamics. This solution is unsatisfactory from both theoretical and empirical points of view. Aside from being non-mechanistic, it introduces additional parameters that lack clear biological meanings and therefore cannot be measured directly. Additionally, this solution still fails to permit the models to address evidence related to population structure.
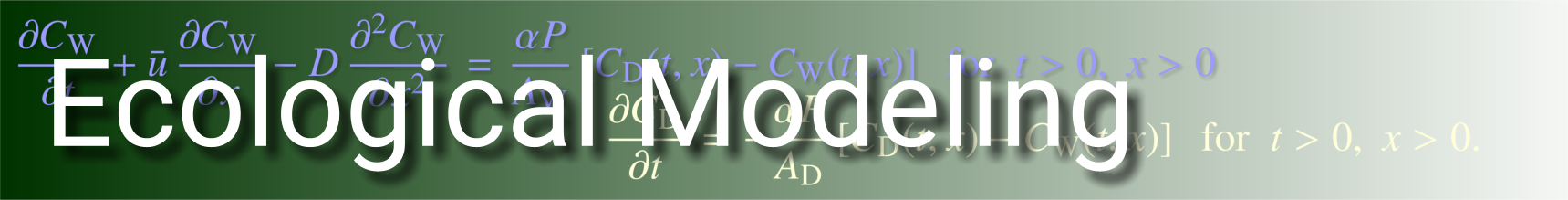
My own approach to improving the theory of the rotifer chemostat is to build new types of models that include basic aspects of population structure and that link these aspects to physiological mechanisms underlying growth and reproduction. The rudiments of this approach are outlined by McNair et al. (1998), who present a model prototype and examples of its dynamics. The model includes two levels of structure: discrete ontogenetic stages (eggs and free-swimming rotifers) and continuous within-stage structure (generalized age in eggs, body size in free-swimming rotifers). Basic physiological processes such as ingestion, assimilation, respiration, and size-dependent differential allocation to somatic versus reproductive mass are explicitly included in the model and form a mechanistic basis for changes in rotifer population size and structure. The model is able to address gross population properties (e.g., total mass, total number of individuals) as well as key observable properties of population structure (e.g., size structure). Numerical examples presented by McNair et al. (1998) indicate that the model has promise, since its qualitative properties were found to agree reasonably well with observed dynamics of total mass and the size structure, and with the observed pattern of differences in the steady-state size distribution at different dilution rates. A more-technical overview of the model can be found here (PDF).
Selected References
McNair, J.N., Boraas, M.E., and Seale, D.B. 1998. Size-structure dynamics of the rotifer chemostat: a simple physiologically structured model. Hydrobiologia 387/388: 469-476.
Boraas, M.E., Seale, D.B., Boxhorn, J.E., and McNair, J.N. 1998. Rotifer size distribution changes during transient phases in open cultures. Hydrobiologia 387/388: 477-482.